Supercomputer-based scientific evidence for safer and better therapeutics, informed investment decisions and greater reach
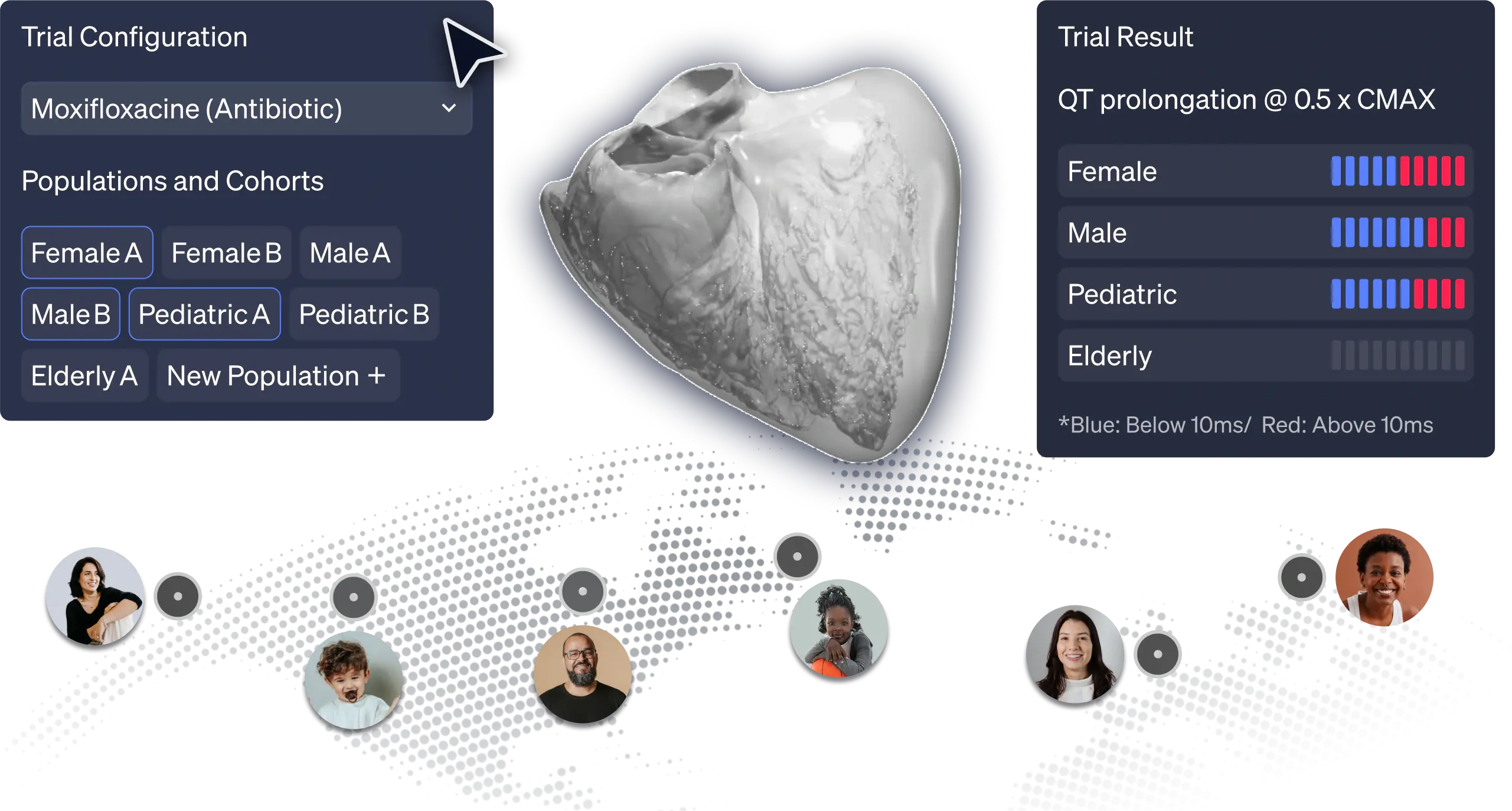
Real trials with Virtual Humans
Integrate Virtual Human Populations to optimize trial design, or when risk levels are high, access to real patients is complex and when ethics prevails
Scientific evidence for Critical decisions
Protect the interest of your biomedical company and your patients, with scientific evidence driven from real-world population data
Value-Driven Healthcare
Refine patient eligibility criteria with new computational biomarkers to drive efficiency, outcome-based reimbursements & value-based health
Control R&D/ product Risks
Maximize returns by anticipating risks, controlling costs and optimizing market reach through stronger statistical significance in your trials results
ELEM's predictive models help pharmaceutical companies assess drug performance across diverse populations, identifying patient and treatment risks early. Integrating Virtual Human Trials early in drug development reduces late-stage failures, enhances patient safety, and minimizes reliance on animal testing.

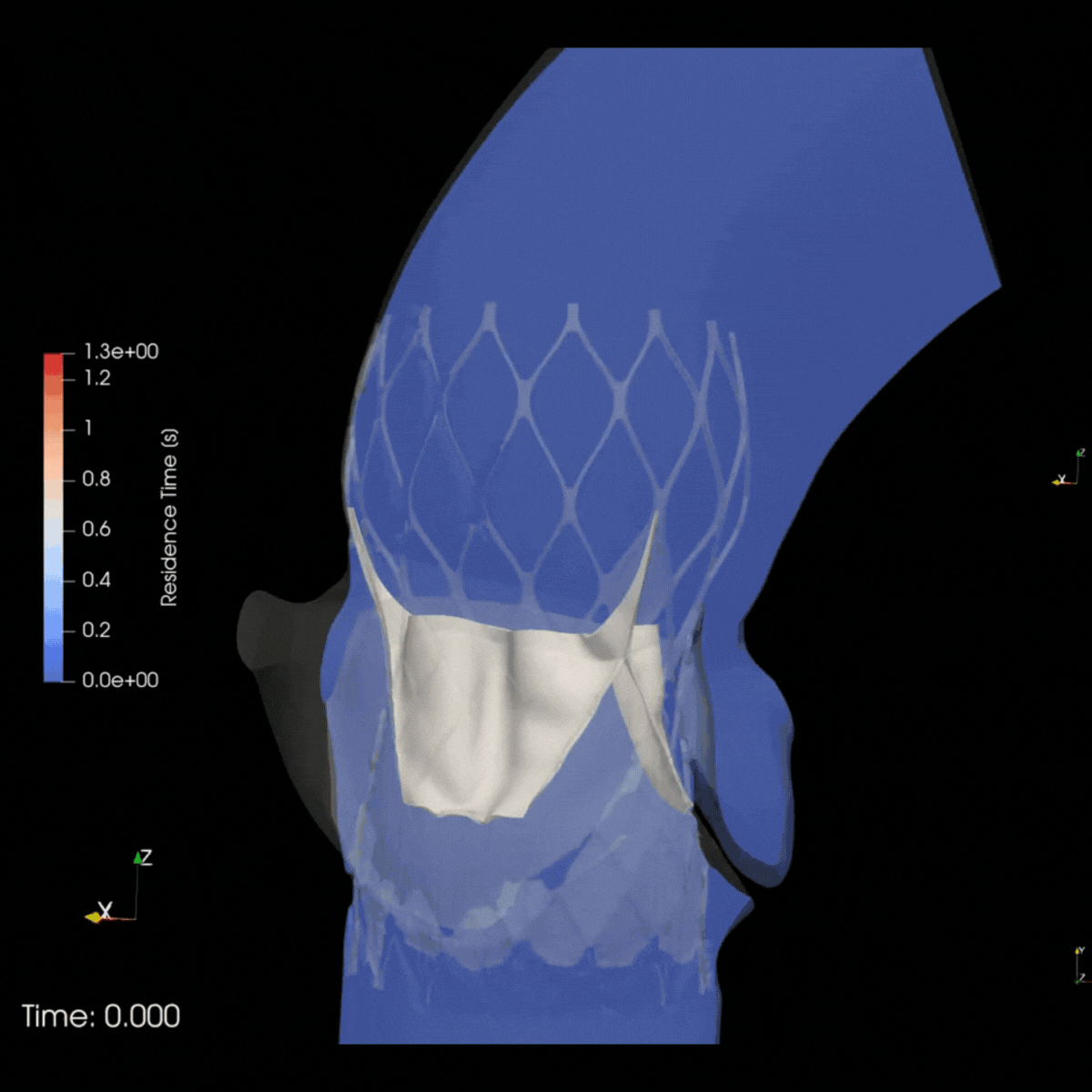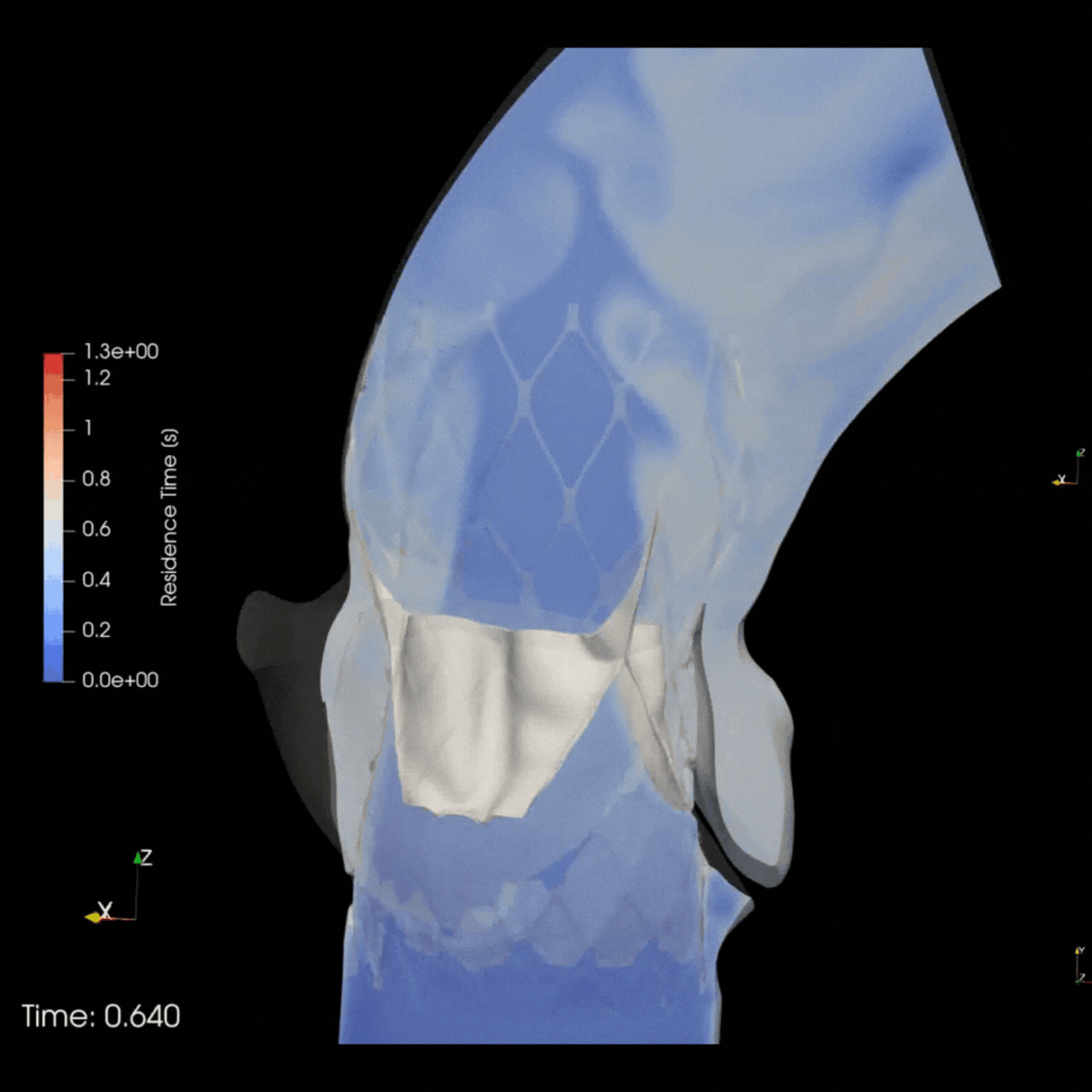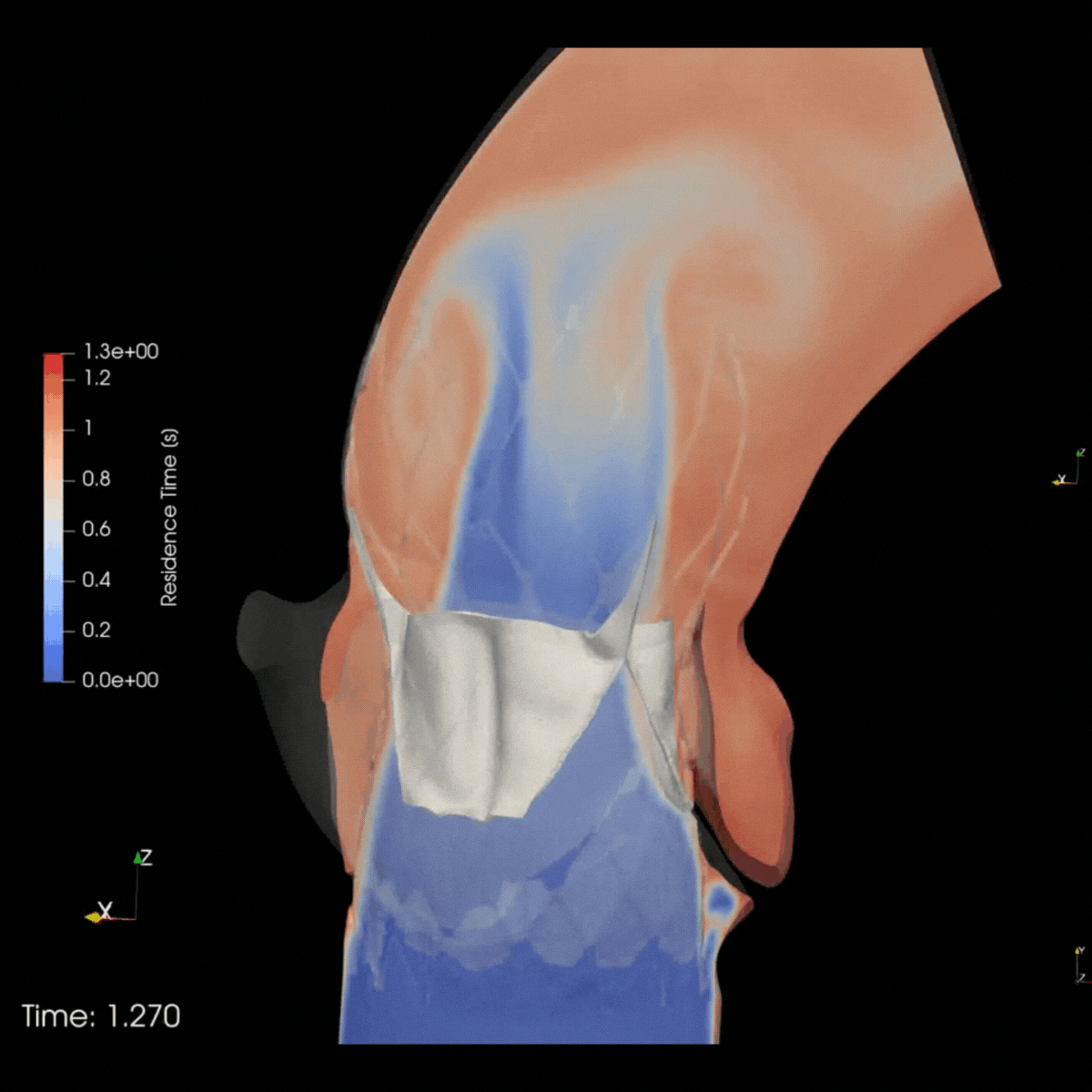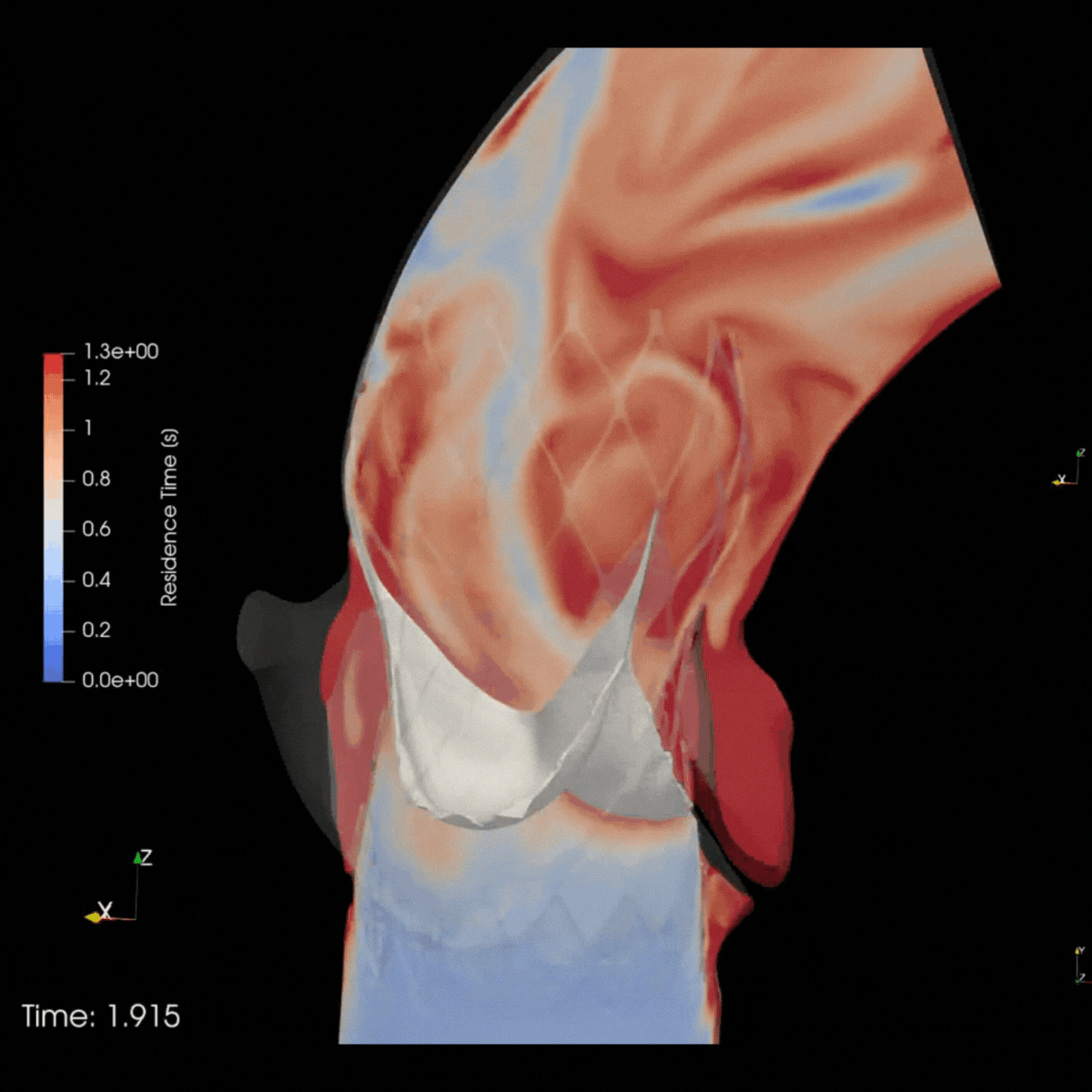
ELEM Virtual Human Populations reflect multiple patient & therapeutic scenarios, providing early insights into product safety and effectiveness. Virtual trials for high-risk devices generate precise evidence and avoid invasive procedures. This reduces patients risks and leads to positive outcomes for clinicians and companies.
Safety first. ELEM’s standardized, comparable and repeatable trials with Virtual Humans enable regulators to compare and validate studies independently, faster and at lower cost. This increases the regulator’s capacity to process new treatments and accounts for patient groups with unmet needs or rare diseases.
CROs adopting ELEM technology develop stronger relationships with sponsors by offering innovative solutions to mitigate trial delays, offer guarantees about inclusion & exclusion criteria and explain outliers. CROs additionally leverage Virtual Humans Populations for effective trial protocols and faster trials.
Safely deploying ELEM Virtual Humans in the clinic helps doctors gain insights into patient responses to treatments whilst increasing patient engagement with visual information. Informed clinical decisions enable HDOs to optimize workflows with efficient cost models for best patient outcomes.
Solutions
Hit-to-lead optimization
Taking into account molecule properties, assay variability, base PK/PD and binding properties (PPB), ELEM V.HEART-Discovery platform is helping our clients screen high volumes of molecules (HTS) up to 100x effective concentrations. Early in-human QT prolongation profiling of the molecule over an extended range, massively reduces the risk during Drug Development.

Paediatrics patient scenarios
From 15 patients’ real data, ELEM studied 10 different pacing scenarios for each of the patients, using its virtual human technology. This enables a MedTech to assess the efficacy of the therapies of their devices, generating the data to eventually support its filing. Additionally, ELEM created, from these virtual twins a population of synthetic 150 more patients to “fill the gap” of the study.
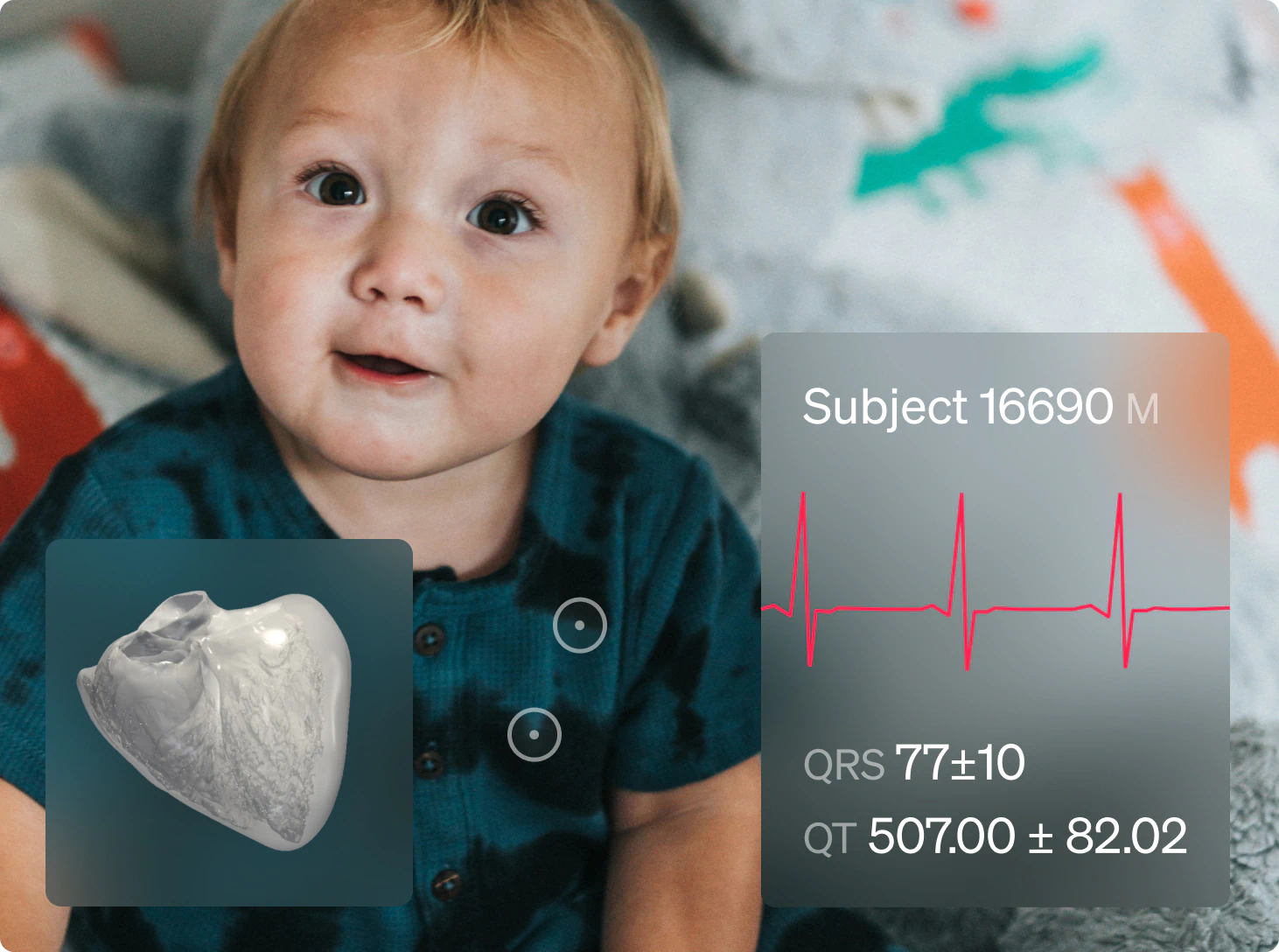
Safety trial in Cardio oncology
ELEM V.HEART platform provided the client with a 5x matching virtual cardio-oncology cohort of 120 gender-balanced Virtual Patients. Predictive results delivered within days allowed the clinical team to prevent adverse events, avoid recruitment delays, and enhance trial protocols.
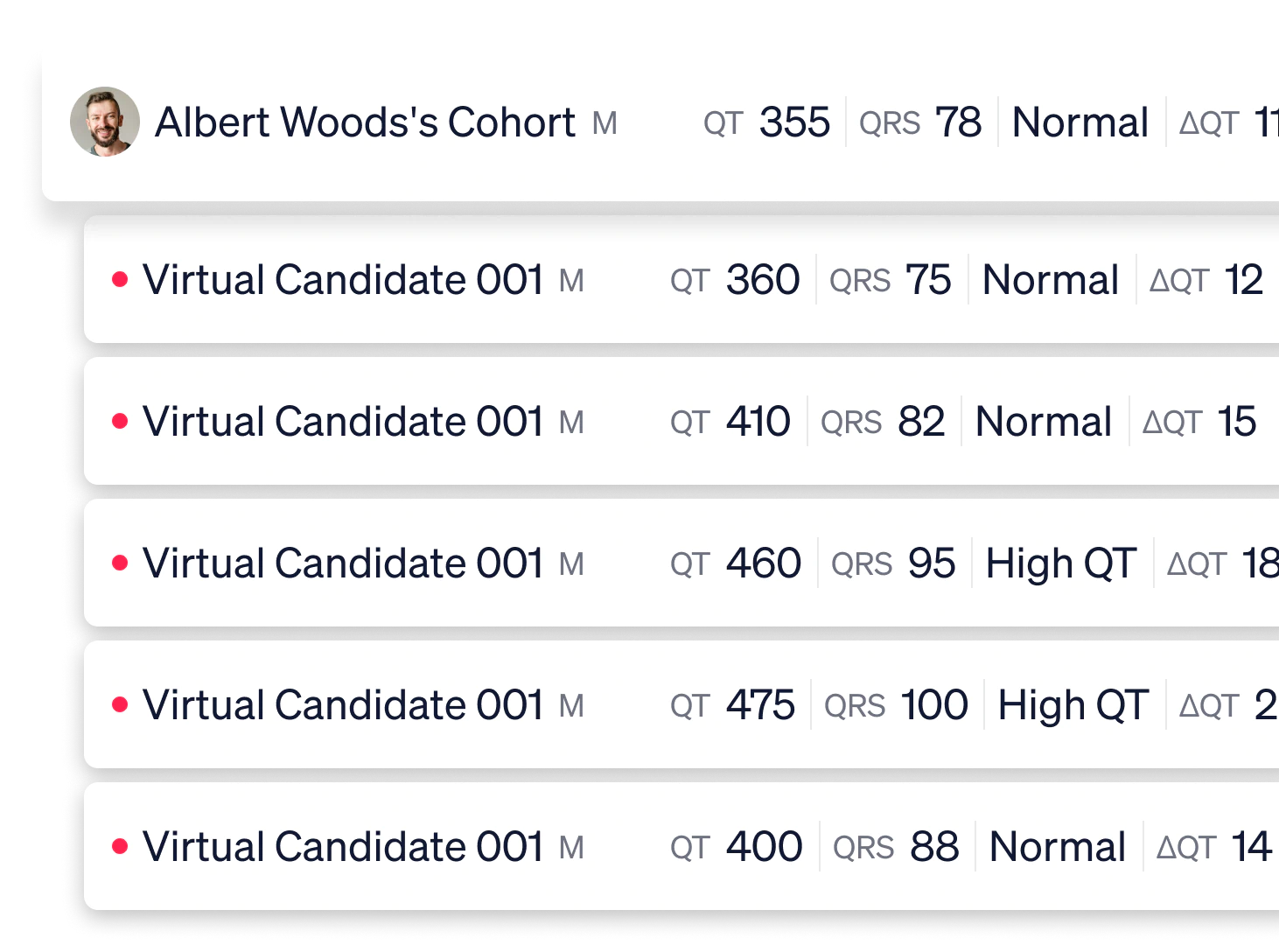
Effective targeting in respiratory
Based on 100 detailed human anatomies of the respiratory system, we create a virtual population of 5000 representing a real population. Our client analyzed and optimized drug delivery to the target area for maximum efficacy —the studies performed in days guaranteed the project timeline was respected with no delays.
+50
experts
developing our technology. Renowned medical, academic, and healthcare specialists
30%
risk reduction
lowering failure rates with advanced cardiac safety predictions.
80%
cost savings
reducing R&D expenses by identifying cardiac risks earlier.
15K
studies
Virtual human studies
800
million hours
dedicated to scientific testing
+150
scientific papers
supporting our technology
ELEM Technology
ELEM Virtual Humans capture medical knowledge. They reduce reliance on animal testing, improve access for underserved groups, and eliminate biases and ethical concerns. Simple to use, this leads to increased profit margins for businesses, cheaper and more effective treatments for patients.
Generate stronger evidence, deliver safe and effective treatments
Virtual Human Twins stem from the vision set by the regulators, FDA and EMA to generate stronger evidence from trials and to deliver safe and effective treatments to every patient.
Unparalleled predictive insights for treatment responses evaluation.
Combining biomedical science, multiscale and multiphysics mechanistic modelling, AI, and supercomputer power, we generate unparalleled predictive insights for treatment responses evaluation.
Accurately replicates working human organs
ELEM’s Virtual Humans technology accurately replicates working human organs (cells, tissue, physiology), healthy and diseased, starting from real anatomical and medical patient data.
